Introducing :Acid -
Base Indicators
The most
common method to get an idea about the pH of solution is to use an acid base
indicator. An indicator is a large organic molecule that works somewhat like a
" color dye". Whereas most dyes do not change color with the amount
of acid or base present, there are many molecules, known as acid - base
indicators , which do respond to a change in the hydrogen ion
concentration. Most of the indicators are themselves weak acids.
The most
common indicator is found on "litmus" paper. It is red below pH 4.5
and blue above pH 8.2.
Color
|
Blue
Litmus
|
Red
Litmus
|
Acid
|
turns
red
|
stays
same
|
Base
|
stays
same
|
turns
blue
|
Other
commercial pH papers are able to give colors for every main pH unit. Universal
Indicator, which is a solution of a mixture of indicators is able to also
provide a full range of colors for the pH scale.
A variety
of indicators change color at various pH levels. A properly selected acid-base
indicator can be used to visually "indicate" the approximate pH of a
sample. An indicator is usually some weak organic acid or base dye that changes
colors at definite pH values. The weak acid form (HIn) will have one color and
the weak acid negative ion (In-) will have a different color. The
weak acid equilibrium is:
HIn
--> H+ + In-
For phenolphthalein: pH 8.2 = colorless; pH 10 = red
For phenolphthalein: pH 8.2 = colorless; pH 10 = red
For
bromophenol blue: pH 3 = yellow; pH 4.6 = blue
See the graphic for more indicators, colors, and
pH ranges.
Explain
the color indicator change:
Use
equilibrium principles to explain the color change for phenolphthalein at the
end of the demonstration.
Solution:
The
simplified reaction is: H+ + OH- --> HOH
As OH- ions
are added, they are consumed by the excess of acid already in the beaker as
expressed in the above equation. The hydroxide ions keep decreasing and the
hydrogen ions increase, pH decreases.
See lower
equation: The indicator equilibrium shifts left, In- ions
decrease. Below pH 8.2 the indicator is colorless. As H+ ions
are further increased and pH decreases to pH 4-5, the indicator equilibrium is
effected and changes to the colorless HIn form.
Equilibrium: HIn --> H+ + In-colorless red
Molecular
Basis for the Indicator Color Change:
Color
changes in molecules can be caused by changes in electron confinement. More
confinement makes the light absorbed more blue, and less makes it more red.
How are
electrons confined in phenolphthalein? There are three benzene rings in the
molecule. Every atom involved in a double bond has a p orbital which can
overlap side-to-side with similar atoms next to it. The overlap creates a 'pi
bond' which allows the electrons in the p orbital to be found on either bonded
atom. These electrons can spread like a cloud over any region of the molecule
that is flat and has alternating double and single bonds. Each of the benzene
rings is such a system.
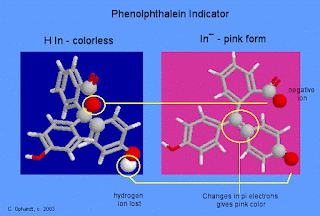
See the
far left graphic - The carbon atom at the center (adjacent to the yellow
circled red oxygen atom) doesn't have a p-orbital available for pi-bonding, and
it confines the pi electrons to the rings. The molecule absorbs in the
ultraviolet, and this form of phenolphthalein is colorless.
In basic
solution, the molecule loses one hydrogen ion. Almost instantly, the five-sided
ring in the center opens and the electronic structure around the center carbon
changes (yellow circled atoms) to a double bond which now does contain pi
electrons. The pi electrons are no longer confined separately to the three
benzene rings, but because of the change in geometry around the yellow circled
atoms, the whole molecule is now flat and electrons are free to move within the
entire molecule. The result of all of these changes is the change in color to
pink.
Naturally occurring
pH indicators[edit]
Many plants or plant parts contain
chemicals from the naturally colored anthocyanin family of
compounds. They are red in acidic solutions and blue in basic. Anthocyanins can
be extracted with water or other solvents from a multitude of colored plants or
plant parts, including from leaves (red cabbage);
flowers (geranium, poppy, or rose petals);
berries (blueberries, blackcurrant);
and stems (rhubarb). Extracting anthocyanins from household plants,
especially red cabbage,
to form a crude pH indicator is a popular introductory chemistry demonstration.
Litmus, used by
alchemists in the Middle Ages and still readily available, is a naturally occurring
pH indicator made from a mixture of lichen species,
particularly Roccella tinctoria.
The word litmus is literally from 'colored moss' in Old Norse (see Litr).
The color changes between red in acid solutions and blue in alkalis. The term
'litmus test' has become a widely used metaphor for any test that purports to
distinguish authoritatively between alternatives.
Hydrangea macrophylla flowers can change color depending on soil acidity. In
acid soils, chemical reactions occur in the soil that make aluminium available
to these plants, turning the flowers blue. In alkaline soils, these reactions
cannot occur and therefore aluminium is not taken up by the plant. As a result,
the flowers remain pink.
Indicator
|
Low pH color
|
High pH color
|
Blue
|
pink to purple
|
|
Red
|
blue
|
|
Red
|
blue
|
Indicators
Indicators are substances whose solutions change
color due to changes in pH. These are called acid-base indicators. They are
usually weak acids or bases, but their conjugate base or acid forms have
different colors due to differences in their absorption spectra.
Do
you know that the color of hydrangea flower depends on the pH of the soil in
which it is grown? This picture shows various colors of hydrangea flowers.
HIn = H+ + In-,
and define the
equilibrium constant as Kai,
[H+][In-]
Kai =
----------.
[HIn]
Which can be rearranged
to give
[In-] Kai
------- = -----
[HIn] [H+]
When [H+] is greater than 10 Kai, In- color dominates, whereas color due
to HIn dominates if [H+] < Kai / 10. The above
equation indicates that the color change is the most sensitive when [H+] = Kai in numerical
value.
We define pKai =
- log(Kai), and the pKai value is also
the pH value at which the color of the indicator is most sensitive to pH
changes.
Taking the negative log
of Kai gives,
[In-]
-log Kai
= -log[H+] - log------
[HIn]
or
[In-]
pH = pKai
+ log-----
[HIn]
This is a very important
formula, and its derivation is very simple. Start from the definition of the
equilibrium constant K, you can easily derive it. Note that pH = pKai when [In-] = [HIn]. In other words, when
the pH is the same as pKai, there are equal
amounts of acid and base forms. When the two forms have equal concentration,
the color change is most noticeable.
Colors of substances
make the world a wonderful place. Because of the colors and structures,
flowers, plants, animals, and minerals show their unique characters.
Many indicators are
extracted from plants. For example, red cabbage juice and tea pigments show
different colors when the pH is different. The color of tea darkens in a basic
solution, but the color becomes lighter when lemon juice is put into a tea. Red
cabbage juice turns blue in a basic solution, but it shows a distinct red color
in an acidic solution.
Some Common Indicators
Name
|
Acid
Color
|
pH Range
of
Color Change |
Base
Color
|
Methyl violet
|
Yellow
|
0.0 -
1.6
|
Blue
|
Thymol blue
|
Red
|
1.2 -
2.8
|
Yellow
|
Methyl orange
|
Red
|
3.2 -
4.4
|
Yellow
|
Bromocresol green
|
Yellow
|
3.8 -
5.4
|
Blue
|
Methyl red
|
Red
|
4.8 -
6.0
|
Yellow
|
Litmus
|
Red
|
5.0 -
8.0
|
Blue
|
Bromothymol blue
|
Yellow
|
6.0 -
7.6
|
Blue
|
Thymol blue
|
Yellow
|
8.0 -
9.6
|
Blue
|
Phenolphthalein
|
Colorless
|
8.2 -
10.0
|
Pink
|
Thymolphthalein
|
Colorless
|
9.4 -
10.6
|
Blue
|
Alizarin yellow R
|
Yellow
|
10.1 -
12.0
|
Red
|
Some common indicators
and their pKai (also referred to as pKa)
values are given in a table form. Since the table is an HTML file, we can not
include the table in the DOS version, but the HTML version allows you to see
this table below:
There is a separate file
for this, and it can also be accessed from the Chemical Handbook menu.
What is Titration?
The word titration comes from the Latin word "titulus",
which means inscription or title. The French word title means rank. Therefore,
Titration means the determination of concentration or rank of a solution with
respect to water with a pH of 7.
The standard solution is usually added from a graduated vessel
called a burette. The process of adding standard solution until the reaction is
just complete is termed as titration and the substance to be determined is said
to be titrated.
All chemical reactions cannot be considered as titrations. A
reaction can serve as a basis of a titration procedure only if the following
conditions are satisfied:
1.
The reaction must be a fast one.
2.
It must proceed stoichiometrically.
3.
The change in free energy (ΔG) during the reaction must be
sufficiently large for spontaneity of the reaction.
4.
There should be a way to detect the completion of the reaction.
End point and Equivalent
point:
For a reaction, a stage which shows the completion of a particular
reaction is known as end point. Equivalence point is a stage in which the
amount of reagent added is exactly and stoichiometrically equivalent to the
amount of the reacting substance in the titrated solution. The end point is
detected by some physical change produced by the solution, by itself or more
usually by the addition of an auxiliary reagent known as an 'indicator'. The
end point and the equivalence point may not be identical. End point is usually
detected only after adding a slight excess of the titrant. In many cases, the
difference between these two will fall within the experimental error.
Indicator:
It is a chemical reagent used to recognize the attainment of end
point in a titration. After the reaction between the substance and the standard
solution is complete, the indicator should give a clear colour change.
When a titration is carried out, the free energy change for the
reaction is always negative.
That is, during the initial stages of the reaction between A & B, when the titrant A is added to B the following reaction takes place.
That is, during the initial stages of the reaction between A & B, when the titrant A is added to B the following reaction takes place.
Equilibrium constant,
a = activity co-efficient.
Large values of the equilibrium constant K implies that the
equilibrium concentration of A & B are very small at the equivalence point.
It also indicates that the reverse reaction is negligible and the product C
& D are very much more stable than the reactants A & B. Greater the
value of K the larger the magnitude of the negative free energy change for the
reaction between A & B. Since,
Where,
R = Universal gas Constant = 8.314 JK-1mol-1,
T = Absolute Temperature.
T = Absolute Temperature.
The reaction of the concentration of A & B leads to the
reduction of the total free energy change. If the concentrations of A & B
are too low the magnitude of the total free energy change becomes so small and
the use of the reaction for titration will not be feasible.
Expressions of Concentration
of Solutions:
The concentration or strength of solution means the amount of
solute present in a given amount of the solution. The concentration may be
expressed in physical or chemical units.
1.
Normality (N): It is
defined as number of gram equivalents of the solute present in 1 litre
(1000mL.) of the solution. If W g of solute of equivalent weight E is present
in V mL of the solution, the normality of the solution is given by:
2.
Molarity (M): It is
defined as the number of moles of the solute present in 1 litre (or 1000 mL) of
the solution. A one molar solution contains 1 mole of the solute dissolved in 1
litre of the solution.
3.
Molality (m): It is
defined as the number of moles of solute dissolved in 1000 g of the solvent.
One molal solution contains one mole of the solute dissolved in 1000 g of the
solvent.
Normal solution:
A solution containing one gram equivalent weight of the solute
dissolved per litre is called a normal solution; e.g. when 40 g of NaOH are
present in one litre of NaOH solution, the solution is known as normal (N)
solution of NaOH. Similarly, a solution containing a fraction of gram equivalent
weight of the solute dissolved per litre is known as subnormal solution. For
example, a solution of NaOH containing 20 g (1/2 of g eq. wt.) of NaOH
dissolved per litre is a sub-normal solution. It is written as N/2 or 0.5 N
solution.
Formulae used in solving numerical problems on volumetric
analysis;
1.
Strength of solution = Amount of substance in g litre-1.
2.
Strength of solution = Amount of substance in g moles litre-1.
3.
Strength of solution = Normality × Eq. wt. of the solute =
molarity × Mol. wt. of solute.
4.
Molarity = Moles of solute/Volume in litre.
5.
Number of moles = Wt.in g/Mol. wt = M × V (initial) = Volume in
litres/22.4 at NTP (only for gases).
6.
Number of milli moles = Wt. in g × 1000/mol. wt. = Molarity ×
Volume in mL.
7.
Number of equivalents= Wt. in g/Eq. wt = x × No. of moles ×
Normality × Volume in litre (Where x = Mol. wt/Eq. wt).
8.
Number of mill equivalents (meq.) = Wt. in g × 1000 / Eq. wt =
normality × volume in mL.
9.
Normality = x × No. of mill moles (Where x = valency or change in
oxi. number).
10.
Normality formula, N1V1 = N2V2, (Where N1,
N2 →
Normality of titrant and titrate respectively, V1, V2 → Volume of titrant and
titrate respectively).
11.
% by weight = Wt. of solvent/Wt. of solution × 100 .
A solution is a homogeneous mixture of two or more components, the
composition of which may be changed. The substan3333333ce which is present in
smaller proportion is called the solute, while the substance present in large
proportion is called the solvent.
Volumetric Analysis:
It involves the estimation of a substance in solution by
neutralization, precipitation, oxidation or reduction by means of another
solution of accurately known strength. This solution is known as standard
solution.
Volumetric analysis depends on measurements of the volumes of
solutions of the interacting substances. A measured volume of the solution of a
substance A is allowed to react completely with the solution of definite
strength of another substance B. The volume of B is noted. Thus we know the
volume of the solutions A and B used in the reaction and the strength of
solution B; so the strength of the other solution A is obtained. The amount (or
concentration) of the dissolved substance in volumetric analysis is usually
expressed in terms of normality. The weight in grams of the substance per litre
of the solution is related to normality of the solution as,
Weight of the substance (g per litre) = Normality × gram
equivalent weight of the substance.
Conditions of Volumetric
Analysis:
i) The
reaction between the titrant and titrate must be expressed.
ii) The reaction should be practically instantaneous.
iii) There must be a marked change in some physical or chemical property of the solution at the end point.
iv) An indicator should be available which should sharply define the end point.
ii) The reaction should be practically instantaneous.
iii) There must be a marked change in some physical or chemical property of the solution at the end point.
iv) An indicator should be available which should sharply define the end point.
Different methods to
determine the endpoint include:
·
pH indicator:
A pH indicator is a substance that it changes its colour in
response to a chemical change. An acid-base indicator changes its colour
depending on the pH (e.g., phenolphthalein). Redox indicators are also
frequently used. A drop of indicator solution is added to the titration at the
start; at the endpoint has been reached the colour changes.
·
A potentiometer
It is an instrument that measures the electrode potential of the
solution. These are used for titrations based on a redox reaction; the
potential of the working electrode will suddenly change as the endpoint is
reached.
·
pH meter:
It is a potentiometer that uses an electrode whose potential depends
on the amount of H+ ion present in the solution. (It is an example of an
ion-selective electrode.) This allows the pH of the solution to be measured
throughout the titration. At the endpoint, there will be a sudden change in the
measured pH. This method is more accurate than the indicator method and is very
easily automated.
·
Conductance:
The conductivity of a solution depends on the ions present in it.
During many titrations, the conductivity changes significantly. (i.e., during
an acid-base titration, the H+ and OH- ions
react to form neutral H2O, this changes the conductivity of the
solution.) The total conductance of the solution also depends on the other ions
present in the solution, such as counter ions. This also depends on the
mobility of each ion and on the total concentration of ions that is the ionic
strength.
·
Colour change:
In some reactions, the solution changes colour without any added
indicator. This is often seen in redox titrations, for instance, when the
different oxidation states of the product and reactant produce different
colours.
·
Precipitation:
In this type of titration the strength of a solution is determined
by its complete precipitation with a standard solution of another substance.
eg:
Acid base titration:
The chemical reaction involved in acid-base titration is known as
neutralisation reaction. It involves the combination of H3O+ ions with OH- ions to
form water. In acid-base titrations, solutions of alkali are titrated
against standard acid solutions. The estimation of an alkali solution using a
standard acid solution is called acidimetry.
Similarly, the estimation of an acid solution using a standard alkali solution
is called alkalimetry.
The Theory of Acid–Base
Indicators:
Ostwald, developed a theory of acid base indicators which gives an
explanation for the colour change with change in pH. According to this theory,
a hydrogen ion indicator is a weak organic acid or base. The undissociated
molecule will have one colour and the ion formed by its dissociation will have
a different colour.
Let the indicator be a weak organic acid of formulae HIn. It has
dissociated into H+ and In- . The unionized molecule has one colour,
say colour (1), while the ion, In- has a different colour, say colour (2). Since HIn and In- have different colours, the actual colour
of the indicator will dependent upon the hydrogen ion concentration [H+].
When the solution is acidic, that is the H+ ions present in excess, the indicator will show predominantly
colour (1). On other hand, when the solution is alkaline, that is, when OH- ions present in excess, the H+ ions furnished by the indicator will be taken out to form
undissociated water. Therefore there will be larger concentration of the ions,
In-. thus the indicator will show predominantly colour (2).
Some indicators can be used to determine pH because of their
colour changes somewhere along the change in pH range. Some common indicators
and their respective colour changes are given below.
Indicator
|
Colour on Acidic Side
|
Range of Colour Change
|
Colour on Basic Side
|
Methyl Violet
|
Yellow
|
0.0 - 1.6
|
Violet
|
Bromophenol Blue
|
Yellow
|
3.0 - 4.6
|
Blue
|
Methyl Orange
|
Red
|
3.1 - 4.4
|
Yellow
|
Methyl Red
|
Red
|
4.4 - 6.2
|
Yellow
|
Litmus
|
Red
|
5.0 - 8.0
|
Blue
|
Bromothymol Blue
|
Yellow
|
6.0 - 7.6
|
Blue
|
Phenolphthalein
|
Colourless
|
8.3 - 10.0
|
Pink
|
Alizarin Yellow
|
Yellow
|
10.1 - 12.0
|
Red
|
i.e., at pH value below 5, litmus is red; above 8 it is blue.
Between these values, it is a mixture of two colours.
Indicators Used for Various
Titrations:
1. Strong Acid against a
Strong Base:
Let us consider the titration of HCl and NaOH. The pH values of
different stages of titration shows that, at first the pH changes very slowly
and rise to only about 4. Further addition of such a small amount as 0.01 mL of
the alkali raises the pH value by about 3 units to pH 7. Now the acid is
completely neutralized. Further of about 0.01 mL of 0.1 M NaOH will amount to
adding hydrogen ions and the pH value will jump to about 9. Thus, near the end
point, there is a rapid increase of pH from about 4 to 9.
An indicator is suitable only if it undergoes a change of colour
at the pH near the end point. Thus the indicators like methyl orange, methyl red and
phenolphthalein can show
the colour change in the ph range of 4t0 10. Thus, in strong acid- strong base
titrations, any one of the above indicators can be used.
2. Weak Acid against Strong
Base:
Let us consider the titration of acetic acid against NaOH. The
titration shows the end point lies between pH 8 and 10. This is due to the
hydrolysis of sodium acetate formed. Hence phenolphthalein is a suitable indicator as its pH range is 8-9.8. However, methyl
orange is not suitable as its pH range is 3.1 to 4.5.
3. Strong Acid against Weak
Base:
Let us consider the titration ammonium hydroxide against HCl. Due
to the hydrolysis of the salt, NH4Cl, formed during the reaction,
the pH lies in the acid range. Thus, the pH at end point lies in the range of 6
to 4. Thus methyl
orange is a
suitable indicator while phenolphthalein is not suitable.
StrongAcids
|
StrongBases
|
WeakAcids
|
WeakBases
|
HCl
|
NaOH
|
Acetic acid
|
Ammonia
|
HNO3
|
KOH
|
Hydrocyanic acid
|
Magnesium hydroxide
|
HBr
|
etc
|
HF
|
Pyridine
|
H2SO4
|
Oxalic acid
|
Sodium carbonate
|
|
HI
|
Ethanoic acid
|
Potassium carbonate
|
|
HClO4
|
etc
|
etc
|
|
Precipitation Titration:
A titrimetric method based on the formation of a slightly soluble
precipitate is called a precipitation titration. The most important
precipitation process in titrimetric analysis utilizes silver nitrate as the
reagent (Argentimetric process).
Many methods are utilized in determining end points of these
reactions, but the most important method, the formation of a coloured
precipitate will be considered here.
1.
In the titration of a neutral solution of chloride ions with
silver nitrate, a small quantity of potassium chromate solution is added to
serve as the indicator. At the end point the chromate ions combine with silver
ions to form the sparingly soluble brick-red silver chromate. This is a case of
fractional precipitation, the two sparingly soluble salts being AgCl (Ksp = 1.2
x 10-10) and Ag2CrO4 (Ksp = 1.7x10-12).
AgCl is the less soluble salt and initially chloride concentration
is high, hence AgCl will be precipitated. Once the chloride ions are over and with
the addition of small excess of silver nitrate solution brick red colour silver
chromate becomes visible. The titration should be carried out in neutral
solution or in very faintly alkaline solution. i.e. within the pH range 6.5-9.
In acid solutions following reaction occurs.
Consequently the chromate ions concentration is reduced and the
solubility product of silver chromate may not be exceeded. In markedly alkaline
solution, silver hydroxide (Ksp = 2.3 x 108) might be precipitated.
2.
The titration can be carried out with dichlorofluorescein as the
indicator. Dichlorofluorescein is an e6 lo8xample of an adsorption indicator.
Adsorption indicators have the interesting property of changing colour when
they stick (adsorb) to the surface of a precipitate. During the titration the
dichlorofluorescein molecules exist as negatively charged ions (anions) in
solution. As the AgCl precipitate is forming, the excess Cl- ions in the solution form a layer of
negative charge on the precipitate surface. As the equivalence point is reached
and passed, the excess Cl- ions
on the precipitate surface are replaced by excess Ag+ ions, giving the surface
a positive charge. The negatively charged indicator will be attracted to the
positively charged precipitate surface where it absorbs and changes colour. The
suspended precipitate will have a pink tinge because of some premature
displacement of chloride ion by the dichlorofluorescein ion. When the pink
colour starts to persist for slightly longer periods of time, the drip rate is
lowered. The end point is reached when the entire solution turns pink. It is
important that the AgCl precipitate be prevented from coagulation during the
titration. For this reason a small amount of dextrin is added to the solution.
Complexometric Titration:
This type of titration depends upon the combination of ions (other
than H+ and OH-)
to form a soluble ion or compound as in the titration of a solution of a
cyanide with AgNO3.
Principle of Complexometric
Titration:
Complexometric titrations are particularly useful for
determination of a mixture of different metal ions in solution. Ethylene
diamine tetra acetic acid (EDTA), is a very important reagent for complex
formation titrations. EDTA has been assigned the formula II in preference to I
since it has been obtained from measurements of the dissociation constants that
two hydrogen atoms are probably held in the form of zwitter ions.
EDTA behaves as a dicarboxylic acid with two strongly acidic
groups. For simplicity EDTA may be given the formula H4Y, the
disodium salt is therefore Na2H2Y and it has the complex
forming ion H2Y2- in aqueous solution. The reactions with cationsmay be represented
as;
M2+ + H2Y 2-→ MY2- + 2H+
M3+ + H2Y 2-→ MY- + 2H+
M4++ H2Y 2-→ MY + 2H+
M3+ + H2Y 2-→ MY- + 2H+
M4++ H2Y 2-→ MY + 2H+
One gram ion of the complex-forming ion H2Y2- reacts in all cases with one gram ion of the metal. EDTA forms
complexes with metal ions in basic solutions. In acid-base titrations the end
point is detected by a pH sensitive indicator. In the EDTA titration metal ion
indicator is used to detect changes of pM. It is the negative logarithm of the
free metal ion concentration, i.e., pM = - log [M2+]. Metal ion
complexes form complexes with specific metal ions. These differ in colour from
the free indicator and a sudden colour change occurs at the end point. End
point can be detected usually with an indicator or instrumentally by
potentiometric or conductometric (electrometric) method.


No comments:
Post a Comment